
plus one chemistry exam predicted question series Exam oriented Le Chatelier's principle
3. Classification of Elements and Periodicity in Properties 5. States of Matter 9. Hydrogen 10. The s-block elements Plus One (XI) Chemistry Question Bank (Solved) XI Chemistry Question Bank (Solved) by Anil Kumar K L XI Chemistry Question Bank by VHSE career guidance cell Plus One/Plus Two (XI/XII) Chemistry Slide Presentation

Fantastic Ncert Physics Class 11 Pdf In Hindi Photosynthesis Formula And Cellular Respiration
May 5, 2023 by hsslive HSE Kerala Board Syllabus HSSLive Plus One Chemistry Chapter Wise Questions and Answers Pdf Free Download in both English Medium and Malayalam Medium are part of SCERT Kerala Plus One Chapter Wise Questions and Answers.

plus one chemistry previous year important questions chapter 2 YouTube
Students can Download Chapter 1 Some Basic Concepts of Chemistry Questions and Answers, Plus One Chemistry Chapter Wise Questions and Answers helps you to revise the complete Kerala State Syllabus and score more marks in your examinations. Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 1 Some Basic Concepts of Chemistry

Plus One Chemistry Chapter 6 Thermodynamics Part 1 സൂപ്പറായി പഠിക്കാം 🔥🔥 YouTube
1. Some Basic Concepts of Chemistry ┗ Click here 2. Structure of Atom ┗ Click here 3. Classification of Elements and Periodicity in Properties ┗ Click here 4. Chemical Bonding and Molecular Structure ┗ Click here 5. States of Matter ┗ Click here 6. Thermodynamics ┗ Click here

Plus one chemistry Chapter 1 Some basic concept of chemistry Part 1 YouTube
Question 1. Which of the following is a mixture? a) Graphite b) Sodium chloride c) Distilled water d) Steel Answer: d) Steel Question 2. 1 µ g = __________ g [10 -3, 10 -6, 10 -9, 10 -12] ' Answer: 10 -6 Question 3. The number of significant figures in 0.00503060 is __________ . Answer: 6 Question 4.

Plus One Chemistry Guide for HSE, VHSE, CBSE, Open School Based on NCERT Syllabus New Jyothi
Students can download the Plus One Chemistry Chapter Wise Question Papers and Answers PDF from the link provided below: Chemistry Previous Year Question Papers Plus One (+1) Chemistry Previous Year Question Papers Download Plus One (XI) Chapter Wise Previous Year Questions Papers & Answers

Plus One English Chapter Wise Questions & Answers Plus One English Previous Year Question
Give illustration of fusion of ice. Answer: 1. It is the enthalpy change when one mole of a solid is converted into its liquid at its melting point. 2. Enthalpy of fusion of ice is 6 kJ/mol. From this it is clear that 6 kJ of energy is required to convert one mole of ice (18 g) into water at 0°C. Question 2.
Class 12 Chemistry chapterwise previous year questions.(PYQ's) and important topics SOLUTIONS
1 /8 Plus One Chemistry Chapter Wise Important Questions Chapter 1 Some Basic Concepts of Chemistry Question 1. Calculate the number of moles of 02 required to produce 240g of MgO by burning Mg. metal. (Atomic mass: Mg = 24, 0 = 16) (March - 2019) Answer: Mg + 1/2 O → MgO Molecular mass of MgO = 24 + 16 = 40

Plus OneChemistry Focus AreaPrevious Year Questions of Chapter 1RevisionPart3 YouTube
Kerala Plus One Chemistry Chapter Wise Questions and Answers. Chapter 1 Some Basic Concepts of Chemistry. Chapter 2 Structure of Atom. Chapter 3 Classification of Elements and Periodicity in Properties. Chapter 4 Chemical Bonding and Molecular Structure. Chapter 5 States of Matter.

Best learning app in Kerala,India for SSLC, CBSE, Plus One,Plus Two Tuition
September 13, 2019 by hsslive. HSE Kerala Board Syllabus HSSLive Plus One Chapter Wise Questions and Answers Pdf Free Download in both English Medium and Malayalam Medium are part of Plus One Kerala SCERT. Here HSSLive.Guru has given Higher Secondary Kerala Plus One Chapter Wise Questions and Answers based on CBSE NCERT syllabus.

Q13 plus one chemistry exam predicted question series Compounds of s Block Elements YouTube
Chapter wise previous questions & answers of Higher Secondary first year (Plus One) chemistry from 2008 to 2022, prepared by Sri.Anil Kumar K L, HSST, AP HSS, Adichanalloor, Kollam published. Click on the below link to start downloading. 1. Some Basic Concepts of Chemistry Previous Questions Answer Key 2. Atomic Structure Previous Questions

CBSE Class 10 Chemistry Chapter Wise Important Questions and Answers for 202223
May 8, 2023 by hsslive. HSSLive.Guru providing HSE Kerala Board Syllabus HSSLive Higher Secondary SCERT Plus One Study Material, Question Bank, Quick Revision Notes, Chapter Wise Notes, Chapter Wise Previous Year Important Questions and Answers, Previous Year Sample Question Papers with Answers based on CBSE NCERT syllabus in both English.

Plus One Chemistry Chapter 1 Question Discussion Focus Area Based Previous Year Question
If you have any query regarding Higher Secondary Kerala Plus One Chemistry Chapter Wise Questions and Answers based on CBSE NCERT syllabus, drop a comment below and we will get back to you at the earliest. HSSLive Plus One HSSLive Chemistry
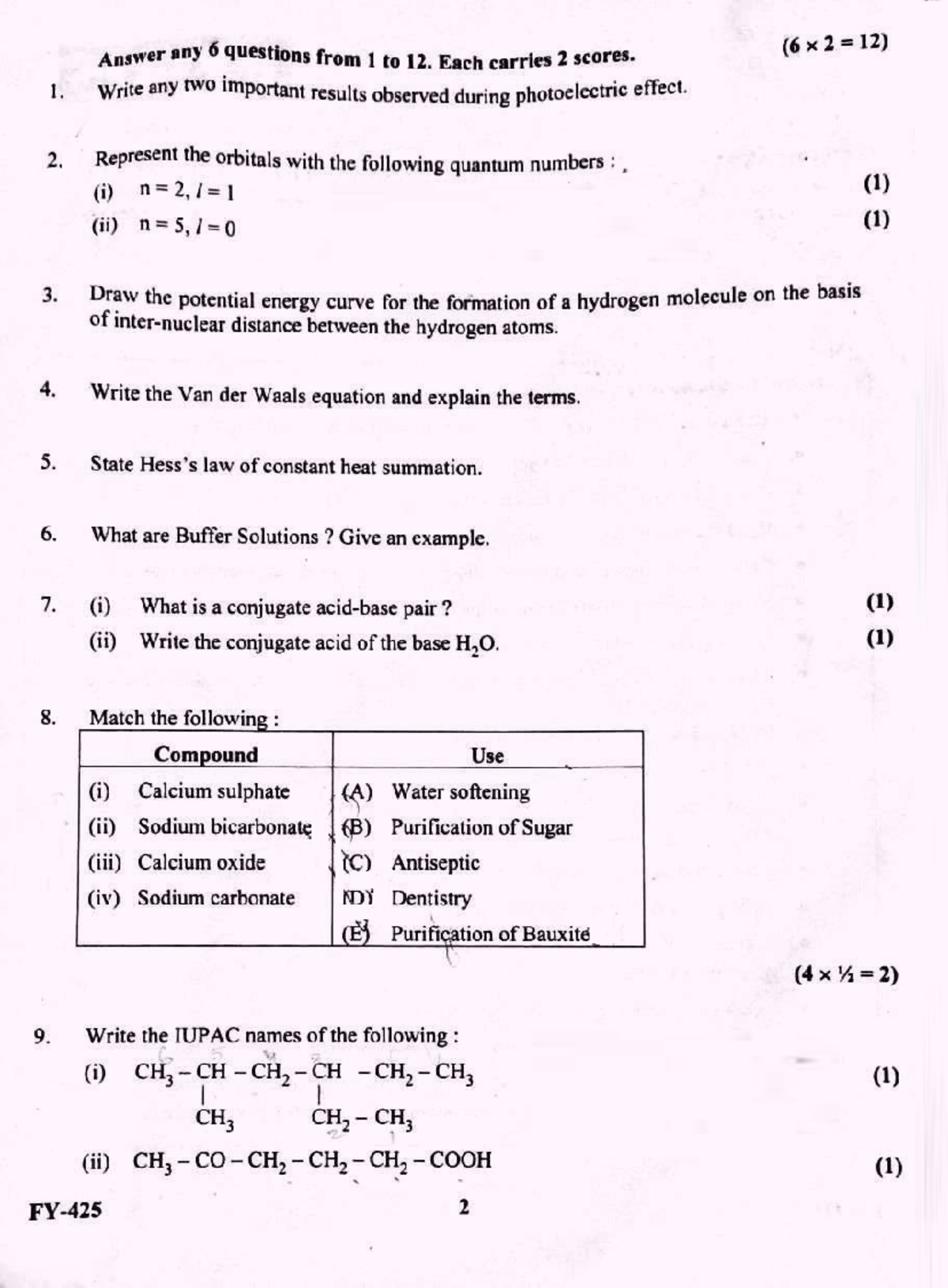
Plus One Improvement Exam 2022 Chemistry Question Paper Chemistry for class 11 Studocu
The Pdf format of the study materials can be downloaded for free from the links provided below.. Chapter No. Chapter Name. 1. Some Basic Concepts of Chemistry. 2. Structure Of The Atom. 3. Classification of Elements and Periodicity in Properties.

Neet Chapter Wise Weightage 2023 Physics Chemistry Biology Gambaran
Chapter 1 The Solid State Chapter 2 Solutions Chapter 3 Electrochemistry Chapter 4 Chemical Kinetics Chapter 5 Surface Chemistry Chapter 6 General Principle and Processes of Isolation of Elements Chapter 7 The p Block Elements Chapter 8 The d and f Block Elements Chapter 9 Coordination Compounds Chapter 10 Haloalkanes and Haloarenes

Plus One Chemistry Quick Revision Part1 Chapters 14 എളുപ്പത്തിൽ പഠിക്കാം.! YouTube
Kerala Plus One Chemistry Chapter Wise Previous Year Questions and Answers. Chapter 1 Some Basic Concepts of Chemistry. Chapter 2 Structure of Atom. Chapter 3 Classification of Elements and Periodicity in Properties. Chapter 4 Chemical Bonding and Molecular Structure. Chapter 5 States of Matter. Chapter 6 Thermodynamics.